Introduction
Obstructive sleep apnoea (OSA) is a chronic sleep disorder in which individuals experience complete or partial obstruction of the upper airway structures,1 2 with a resultant reduction or complete blockage of airflow during sleep, intermittent hypoxia and sleep disturbances.3 Common symptoms of OSA, such as excessive daytime sleepiness, fatigue, heavy snoring and non-refreshing sleep, have the potential to play an influential role in one’s quality of life (QOL).4 OSA also increases one’s risk of developing several cardiovascular-related comorbidities, including heart failure, arrhythmias and coronary artery disease.5 Individuals with an apnoea–hypopnoea index (AHI) >36 have a threefold higher risk of all-cause mortality in comparison to other AHI scores (HR=3.30; 95% CI 1.74 to 6.26).6 Patients with OSA who are classified as moderate (AHI 15–30) to severe (AHI >30) have an increased risk of many adverse outcomes, specifically all-cause mortality.7 As a result of this, the risk of mortality in patients diagnosed with OSA has become a growing and pervasive concern.
OSA is a growing public health concern, with previous studies in the literature reporting an increasing global prevalence of upwards of 1 billion individuals.8 Estimates within the USA suggest that approximately 15% of adults are clinically diagnosed with OSA.9 A large portion of the population suffers from OSA-like symptoms without receiving any diagnosis or treatment. This group continues to further the burden placed on the public health and medical systems. Identifying individuals diagnosed with OSA who are considered at-risk for adverse health outcomes can reduce the economic cost and burden on the healthcare system.10
Existing evidence on the association between OSA and the risk of sudden death is insufficient and inconclusive. Our objective is to estimate the association of OSA and the mortality risk. We hypothesise that OSA is a risk factor for cardiac events and all-cause mortality. The present systematic review and meta-analysis estimate the risk of all-cause and cardiovascular mortality in individuals with OSA.
Abstract
Objectives: Over 1 billion individuals worldwide experience some form of sleep apnoea, and this number is steadily rising. Obstructive sleep apnoea (OSA) can negatively influence one’s quality of life and potentially increase mortality risk. However, the association between OSA and mortality has not been reliably estimated. This meta-analysis estimates the risk of all-cause and cardiovascular mortality in individuals with OSA.
Design Systematic review and meta-analysis.
Data sources MEDLINE, Cochrane Library, Scopus and Joanna Briggs Institute Evidence-Based Practice databases were searched from inception through 1 January 2020.
Eligibility criteria for selecting studies We included observational studies assessing the association of sudden deaths in individuals with and without OSA.
Data extraction and synthesis Two independent reviewers (AES and ESH) extracted data and assessed the risk of bias using the Newcastle-Ottawa Scale quality assessment tool. Data were pooled using the random-effects models and reported as risk ratios (RRs) with 95% CIs. Heterogeneity was quantified with I2 statistic.
Results We identified 22 observational studies (n=42 099 participants). The mean age was 62 years and 64% were men. OSA was associated with all-cause sudden death (RR=1.74, 95% CI: 1.44 to 2.10, I2=72%) and cardiovascular mortality (RR=1.94, 95% CI: 1.39 to 2.70, I2=32%). A marginally significant dose–response relationship between severity of OSA and the risk of death was observed (p for interaction=0.05): mild OSA (RR=1.16, 95% CI: 0.70 to 1.93), moderate OSA (RR=1.72, 95% CI: 1.11 to 2.67) and severe OSA (RR=2.87, 95% CI: 1.70 to 4.85). Meta-regression analysis showed that older age was a significant contributing factor in the relationship between OSA and mortality. The median study methodological quality was considered high.
Conclusions OSA is a significant risk factor for all-cause mortality and cardiac mortality. Prevention and treatment strategies to optimise survival and quality of life in individuals with OSA are urgently needed.
PROSPERO registration number CRD42020164941.
Methods
Search strategy and selection criteria
Design
We registered the present study with PROSPERO and the study protocol has been published.11 The findings are reported according to the guidelines of the of the Meta-analyses Of Observational Studies in Epidemiology checklist (online supplemental table 1).
Supplemental material
Data source and searches
We searched MEDLINE, Cochrane Library, Scopus and Joanna Briggs Institute Evidence-Based Practice databases from inception through 1 January 2020. Full search terms used in this study are presented in online supplemental table 2. In summary, search terms included Medical Subject Headings with combinations of ‘sleep apnoea, obstructive’ and ‘death, sudden and ‘cardiac death’’. We screened reference lists of identified articles for potential eligibility as well. We did not impose any limitations related to the date of publication, language or geographical location.
Study selection
We included observational studies that reported rates of sudden death in individuals with OSA. Studies that reported the rates of sudden death in both patients with and without OSA were included. The exclusion criteria were: (1) studies not conducted on humans and did not report the estimates of the outcome of interest or, (2) did not provide enough information to calculate them, (3) review papers, (4) meta-analyses, (5) literature reviews, (6) commentaries and (7) conference abstracts.
Data extraction
Two authors (ESH and AES) screened titles and abstracts of the studies for inclusion eligibility independently. After screening initial articles, ESH and AES manually screened full-text articles for continued inclusion criteria. Disagreements were settled by another author (PS). Relevant data from each report were extracted and this included first author, year of publication, country of publication, number of participants with and without OSA, the median age of participants in both study groups, the median body mass index of participants with and without OSA, the proportion of the sample that was men, the proportion of the study sample who smoked, the proportion of the study sample with hypertension and diabetes, the number of participants with OSA who died, the number of participants without OSA who died, the number of participants who did not die from both groups, risk ratios (RRs) of sudden death and their corresponding 95% CI. Priority was given to adjusted estimates.
Study quality assessment and categorisation
Study methodological quality was assessed independently by the two reviewers (ESH, AES) using the Newcastle-Ottawa Scale quality assessment tool. Three parameters were used for rating observational studies: selection, comparability between exposed and unexposed groups, and exposure/outcome assessment. Studies with less than 5 stars of quality were given a low rating, those with 5–7 were given moderate quality and greater than 7 was considered high. The risk of bias for each included trial was assessed independently.
Statistical analyses
We summarised the study selection process, the sample size and the sociodemographic characteristics of each study, including sex, mean/median age and the number of variables adjusted. The primary outcome was the risk of all-cause sudden mortality in individuals with OSA compared with individuals without OSA.
Cardiovascular mortality associated with OSA was the secondary outcome of interest. To calculate the pooled effect estimate and the 95% CIs, random-effects models with a generic inverse-variance method were fitted with the metagen function from the R package meta.12 DerSimonian and Laird method was used to calculate the between-study variance.13 We used the reported RR estimates (RR, HR, OR) as measures of the association between OSA and the risk of mortality. If an outcome is rare in all populations and subgroups, the distinctions among different measures of RRs (eg, ORs, rate ratios and RRs) can be ignored,14 thus we combined RRs and HRs with ORs in the present meta-analysis and reported the pooled effect size as RRs as common risk estimates for all studies.
We quantified between-study variation using I2 statistics, expressed as % (low (25%), moderate (50%) and high (75%)).15 16 Influence sensitivity analysis (leave-out-one method) was conducted to estimate the influence of individual study on the overall pooled RR.17 Potential sources of heterogeneity were explored with subgroup and meta-regression analyses. Mean or median age, continent, publication year, the proportion of individuals with diabetes and gender were used as covariates.18 Funnel plots and Egger’s test were conducted to explore publication and small study bias.19 Finally, possible publication bias was adjusted using trim and fill analyses using Duval and Tweedie’s non-parametric method.20 R software, V.3.4.3 (R Core Team, Vienna, Austria) was used to conduct the analyses.21
Results
Overview
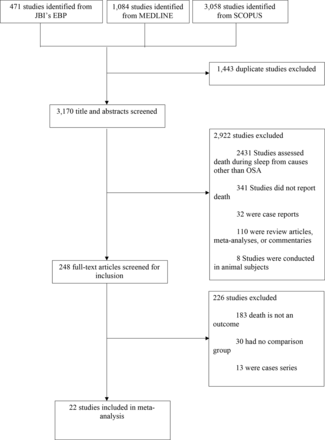
Figure 1 shows the study selection process. We identified 4613 reports of which 22 met eligibility criteria.6 7 22–47 Of the studies, 12 studies were from North America, 5 from Europe, 3 from Asia and 1 from South America and Australia each. A total of 42 099 individuals were included in the final quantitative analysis. The mean age was 62 years and 64% were men. The median study methodological quality score was 9 (7–9) (online supplemental table 3).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. JBI, Joanna Briggs Institute; OSA, obstructive sleep apnoea;EBP, Evidence-Based Practice.
The risk of all-cause sudden death associated with OSA
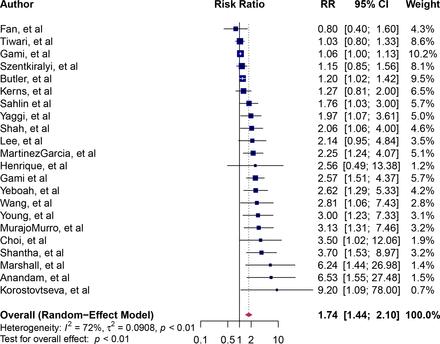
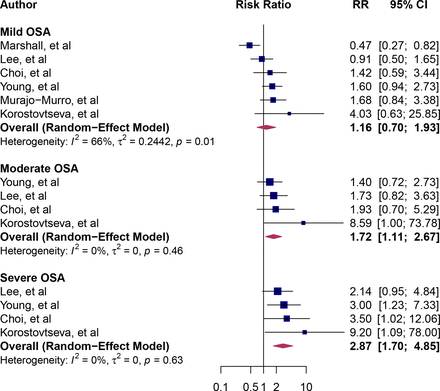
The RR point estimates for all-cause sudden death associated with OSA ranged from 0.80 to 9.20 (figure 2). The pooled RR of sudden death associated with OSA was 1.74 (95% CI: 1.4 to 2.10), implying an approximately twofold higher risk of death. Between-study heterogeneity was moderate (I2=72%). We calculated the dose–response relation between severity OSA and all-cause sudden death. There was a marginally significant dose–response relationship between OSA and all-cause sudden death (p for interaction=0.05). The RR for mild OSA was 1.16 (95% CI: 0.70 to 1.93, I2=66%), for moderate OSA was 1.72 (95% CI: 1.11 to 2.67, I2=0%) and severe OSA was 2.87 (95% CI: 1.70 to 4.85, I2=0%) (figure 3).
Forest plot of pooled risk ratio for the association of OSA with all-cause sudden death. Blue squares and their corresponding lines are the point estimates and 95% CI. Maroon diamonds represent the pooled estimate (width denotes 95% CI). Heterogeneity was considered high (I2=72%). OSA, obstructive sleep apnoea.
Forest plot of subgroup analysis by the severity of OSA. Blue squares and their corresponding lines are the point estimates and 95% CI. Maroon diamonds represent the pooled estimate for each subgroup (width denotes 95% CI). Heterogeneity by severity of OSA: mild (I2=66%); moderate (I2=0%); severe (I2=0%); p for interaction comparing the different subgroups=0.05. OSA, obstructive sleep apnoea.
The risk of cardiovascular mortality associated with OSA
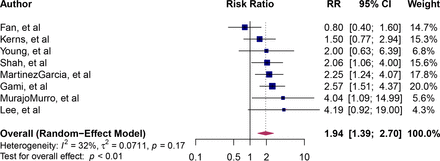
The point estimates of cardiovascular mortality ranged from 0.80 to 4.19 (figure 4). Overall, individuals with OSA had a nearly twofold higher risk of cardiovascular mortality than those without OSA (RR=1.94, 95% CI 1.39 to 2.70, I2=32%).
Forest plot of pooled risk ratio for the association of OSA and cardiovascular mortality. Blue squares and their corresponding lines are the point estimates and 95% CI. Maroon diamonds represent the pooled estimate for each subgroup (width denotes 95% CI). OSA, obstructive sleep apnoea.
Subgroup and meta-regression analyses
The study heterogeneity for the association of OSA and all-cause sudden death was moderate (I2=72%). Therefore, we explored sources of heterogeneity by conducting subgroup analysis with study quality scores and the continent of the study population as covariates. Studies with a score of 9 were compared with those with a score of <9. In the studies with high-quality score, the relationship between OSA and mortality was significant (RR=1.91, 95% CI: 1.50 to 2.44, I2=64%) but not in the studies with a moderate quality score (RR=1.52, 95% CI: 0.97 to 2.37, I2=79%) (figure 5). The test for the subgroup differences was not significant (p=0.37).
Forest plot of subgroup analysis by study quality score. Blue squares and their corresponding lines are the point estimates and 95% CI. Maroon diamonds represent the pooled estimate for each subgroup (width denotes 95% CI). Heterogeneity by methodological quality score: high quality score (I2=64%); moderate quality score (I2=79%); p for interaction comparing the different subgroups=0.37.
Subgroup analysis by continents showed significant pooled RRs for North America, Europe and Australia. However, the pooled RRs for Asia and South America were not significant (figure 6). The test for the subgroup differences was not significant (p=0.47).
Forest plot for subgroup analysis by the continent of the study population. Blue squares and their corresponding lines are the point estimates and 95% CI. Maroon diamonds represent the pooled estimate for each subgroup (width denotes 95% CI). Heterogeneity by continent: North America (I2=77%); Europe (I2=62%); Asia (I2=64%); South America (I2 not applicable); Australia (I2=not applicable); p for interaction comparing the different subgroups=0.47.
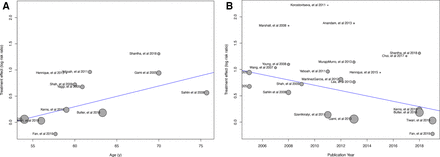
Furthermore, we conducted univariate meta-regression with mean or median study age, publication year, the proportion of participants with diabetes and male proportion as covariates.48 Meta-regression results revealed that year of publication and mean age significantly explained the variations in the pooled estimates (table 1, figure 7). On the other hand, no statistically significant effects from sex or diabetes covariates were observed.
Univariate meta-regression analysis. Meta-regression bubble plots using age (A) and the publication year (B) as covariates.
Results of meta-regression analysis.
Publication bias and influence analysis
We explored publication and small study bias with funnel plots and Egger’s test. We found the publication bias to be significant, as evidenced by asymmetrical funnel plot and significant Egger’s test (p<0.0001) (online supplemental figure 1). To adjust for the publication bias, the trim and fill method was conducted.20 Our analyses showed that if the asymmetry is due to publication bias, the adjusted effect estimates for OSA and sudden death remained significant (RR=1.23, 95% CI: 1.02 to 1.50, p=0.03) (online supplemental figure 2). Finally, influence sensitivity analyses did not indicate outlier studies17 (online supplemental figure 3).
Discussion
The results of the present systematic review and meta-analysis suggest that individuals with OSA have a greater risk of all-cause sudden and cardiovascular death. The risk increases with age.
The twofold higher risk of deaths associated with OSA was similar to the effect estimates by Fonseca and colleagues who summarised studies published between 2002 and 2014 and included 13 394 participants from 13 studies.49 There is a large amount of evidence in research that supports the notion that OSA is associated with numerous cardiovascular conditions, including hypertension, coronary artery disease, congestive heart failure, arrhythmias and more.50 This association may be explained by the influence that the nervous system has on the sleep cycle in humans. OSA results in intermittent hypoxia and oxygen desaturation during sleep, which may cause over-arousal of the central nervous system to increase airflow. The complex relationship between the sympathetic nervous system and the autonomic nervous system causes a transient increase in both systolic and diastolic blood pressure during apnoeas.51 52 Acute sympathetic activation during sleep and sustained elevation in sympathetic activity while awake and are important mechanisms of cardiovascular morbidity and mortality in patients with OSA.53–55
Furthermore, individuals with OSA experience sustained oxidative stress. OSA is associated with a group of proinflammatory and prothrombotic factors that are important in the development of atherosclerosis.56 C reactive protein, an inflammatory marker of oxidative stress, is increased in OSA patients and is associated with endothelial dysfunction.57 Also, Advanced glycation endproducts are increased in non-diabetic subjects with OSA and are associated with the severity of OSA.58 Lastly, fibrinogen, plasminogen activator inhibitor and reduced fibrinolytic activity in OSA is associated with enhanced platelet activity and aggregation, endothelial cells leucocyte adhesion and accumulation leading to atherosclerosis and myocardial infarction.56
Our study had some limitations. First, although we gave priority to adjusted RR, it isplausible that residual confounding persisted in the estimates provided by each study. Therefore, our results should be interpreted with caution. Second, we combined adjusted and unadjusted RR in calculating pooled estimates. It is possible that such a method could have introduced the medium between-study heterogeneity we observed. Nevertheless, we used robust statistical models to explore covariates responsible for the observed variations. Indeed, we found age, study quality, publication year and the continent of the study population to significantly impact the variation of the pooled estimates. Lastly, although this meta-analysis included studies representative of five continents, including North America, Australia, Europe, Asia and South America, we were unable to identify any studies from Africa. Thus, these findings may not be generalisable in the African setting. There is a need for further research to confirm if the association between OSA and mortality remains true in populations not represented in our meta-analysis.
Conclusion
Individuals with OSA have nearly a twofold higher risk of sudden death and cardiovascular mortality. Treatments and interventions related to decreasing this risk and other adverse outcomes are necessary to optimise survival and QOL.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This is a systematic review and meta-analysis, and individual patient was not used. Therefore, we did not need IRB or an ethics board approval.
References
- ↵
- Arnold J,
- Sunilkumar M,
- Krishna V, et al
- ↵
- Osman AM,
- Carter SG,
- Carberry JC, et al
- ↵
- Bonsignore MR,
- Baiamonte P,
- Mazzuca E, et al
- ↵
- Motamedi KK,
- McClary AC,
- Amedee RG
- ↵
- Jean-Louis G,
- Zizi F,
- Clark LT, et al
- ↵
- Yaggi HK,
- Concato J,
- Kernan WN, et al
- ↵
- Marshall NS,
- Wong KKH,
- Cullen SRJ, et al
- ↵
- Benjafield AV,
- Ayas NT,
- Eastwood PR, et al
- ↵
- Young T,
- Palta M,
- Dempsey J, et al
- ↵
- Tarasiuk A,
- Reuveni H
- ↵
- Heilbrunn E,
- Ssentongo P,
- Chinchilli VM, et al
- ↵
- Balduzzi S,
- Rücker G,
- Schwarzer G
- ↵
- DerSimonian R,
- Kacker R
- ↵
- Greenland S
- ↵
- Higgins JPT,
- Thompson SG
- ↵
- Higgins JPT,
- Thompson SG,
- Deeks JJ, et al
- ↵
- Viechtbauer W,
- Cheung MW-L
- ↵
- Schwarzer G,
- Carpenter JR,
- Rücker G
- ↵
- Egger M,
- Davey Smith G,
- Schneider M, et al
- ↵
- Duval S,
- Tweedie R
- ↵
- Team RC
- ↵
- Kerns ES,
- Kim ED,
- Meoni LA, et al
- ↵
- Zhang M,
- Li L,
- Fowler D, et al
- ↵
- Gami AS,
- Olson EJ,
- Shen WK, et al
- ↵
- Martins EF,
- Martinez D,
- da Silva FABS, et al
- ↵
- Szentkiralyi A,
- Czira ME,
- Molnar MZ, et al
- ↵
- Shah NA,
- Yaggi HK,
- Concato J, et al
- ↵
- Sahlin C,
- Sandberg O,
- Gustafson Y, et al
- ↵
- Cassar A,
- Morgenthaler TI,
- Lennon RJ, et al
- ↵
- Gami AS,
- Howard DE,
- Olson EJ, et al
- ↵
- Rössner S,
- Lagerstrand L,
- Persson HE, et al
- ↵
- Tiwari R,
- Lyu B,
- Alagusundaramoorthy S, et al
- ↵
- Sweed RA,
- Hassan S,
- ElWahab NHA, et al
- ↵
- Butler MP,
- Emch JT,
- Rueschman M, et al
- ↵
- Fan J,
- Wang X,
- Ma X, et al
- ↵
- Shantha G,
- Mentias A,
- Pothineni NVK, et al
- ↵
- Jennum P,
- Baandrup L,
- Tønnesen P, et al
- ↵
- Lee J-E,
- Lee CH,
- Lee SJ, et al
- ↵
- Yeboah J,
- Redline S,
- Johnson C, et al
- ↵
- Uchôa CHG,
- Danzi-Soares NdeJ,
- Nunes FS, et al
- ↵
- Anandam A,
- Patil M,
- Akinnusi M, et al
- ↵
- Choi J-W,
- Song JS,
- Lee YJ, et al
- ↵
- Korostovtseva LS,
- Sviryaev YV,
- Zvartau NE, et al
- ↵
- Wang H,
- Parker JD,
- Newton GE, et al
- ↵
- Young T,
- Finn L,
- Peppard PE, et al
- ↵
- Martínez-García M-A,
- Campos-Rodríguez F,
- Catalán-Serra P, et al
- ↵
- Muraja-Murro A,
- Eskola K,
- Kolari T, et al
- ↵
- Borenstein M,
- Hedges LV,
- Higgins JP
- ↵
- Fonseca MIP,
- Pereira T,
- Caseiro P
- ↵
- Lee W,
- Nagubadi S,
- Kryger MH, et al
- ↵
- Bisogni V,
- Pengo MF,
- Maiolino G, et al
- ↵
- Brodovskaya TO,
- Grishina IF,
- Peretolchina TF, et al
- ↵
- Shamsuzzaman AS,
- Somers VK,
- Knilans TK, et al
- ↵
- Somers VK,
- Dyken ME,
- Clary MP, et al
- ↵
- Narkiewicz K,
- Somers VK
- ↵
- Kasasbeh E,
- Chi DS,
- Krishnaswamy G
- ↵
- Volná J,
- Kemlink D,
- Kalousová M, et al
- ↵
- Tan KCB,
- Chow W-S,
- Lam JCM, et al
Supplementary materials
-
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
-
ESH and PS contributed equally.
-
Contributors AES, PS, ESH and VMC conceived the study. AES, ESH and PS conducted the literature search. AES and PS completed data analysis. AES, ESH, PS, VMC and JO interpreted the data. AES, ESH and PS wrote the manuscript. All authors agreed to the manuscript in its final form.
-
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
-
Competing interests None declared.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.